The SmartForm is the series of web-pages (or forms) where you can input specific information about a study. The following section describes what information should go into each space of the SmartForm.
Important! Completing elements of the SmartForm or Clicking “Finish” does not send the study for review. The PI must click Submit (marked with a red arrow in the submission workspace) for the submission to proceed on for the next state of review.
SmartForm Section: Basic Information
1. Title of study. Enter the complete study title. Please avoid use of quotation marks in the study title.
2. Short title. The short title identifies the study throughout the system, such as in your inbox and in an IRB reviewer's list of submissions to review. This should be 80 characters or less, and should not contain quotation marks. If left blank, the system will automatically pull in the first 80 characters of the (long) study title.
3. Brief description or abstract. Enter a brief description of the study or the study abstract. This should be 225 words or less.
4. What kind of study is this?
The options selected here will show additional features in other sections of the system.
Select Multi-Site or Collaborative to allow for the entry/addition of information about study sites and if the project may involve:
• More than one site conducting the study (completely or in part), or
• Collaborators with primary affiliation with other institutions, or
• If it is unclear if there will be additional sites.
Select Single-Site Study if the project only involves work at Harvard, by Harvard affiliated researchers.
5. Will an external IRB act as the IRB of record for this study? Select Yes if you are submitting an application requesting that a Harvard IRB rely on the review of another IRB. Select No if you are requesting that a Harvard IRB review and approve the study.
If you selected Yes, see section: Requesting External IRB Review for additional instruction related to SmartForm page contents and submission requirements.
Important! This item on the SmartForm can only be edited until reliance is confirmed/pre-review is completed by IRB staff. If a selection is made and needs to be changed after reliance is confirmed/pre-review is completed, drafting and review of the submission can be restarted by completing the Copy Submission activity, making additional edits, and submitting the revised study.
6. Will your IRB act as the single IRB of record for any other participating sites? (only displays if question 4 is marked “Collaborative” AND question 5 is marked "no") Select Yes if you are submitting an application requesting that Harvard IRB review on behalf of any other institution or IRB. Select No if you are sure that additional sites will complete their own review of the study.
Note that question 4 must be marked “Collaborative” AND question 5 must be marked “Yes” for sites to be added via the Add/Manage Participating Sites activity and for Harvard to confirm review on behalf of another IRB.
Important! For studies created before 12/15/2017
To manage existing Participating Sites: Revise the listing in the Research Locations (formerly “External Sites”) and Local Site, supporting documents areas of the SmartForm.
To propose any substantive changes to an existing Participating Site or to add new Participating Sites: Revise questions 4 and 5 on the Basic Information page of the SmartForm (during clarifications requested of via a modification to “other parts of the study”) to indicate that this is a collaborative study (at question 4) where you are requesting that Harvard IRB review on behalf of another institution or IRB (at question 5). Once IRB staff review the request, they will work with you to include any additional needed documentation/context for review. After modification approval, you will be able to add the Participating Site via additional activity (Add/Manage Participating Sites), on the main study workspace.
7. Lead Principal Investigator. (only displays if question 4 is marked “Multi-site or Collaborative” AND question 5 is marked “Yes”). The options are limited to individuals with a Harvard ID/HarvardKey. If the Lead Principal Investigator name cannot be found on the list, leave this space blank. For details on who may be listed in this space, please visit your IRB website or Investigator Manual..
8. Local Principal Investigator. Enter the name of the Harvard Principal Investigator. The options are limited to individuals with a Harvard ID/HarvardKey. For details on who may be listed in this space, please visit your IRB website or Investigator Manual.
This field will default to the name of the person completing the form. It may be changed by selecting clear and typing the name in the space provided (a list will appear with names from which to select), or you can click the select option to search the list of names. Use the “%” symbol to replace portions of a name which are unclear or possibly not spelled correctly. This is called a “wildcard” character.
9. Does the investigator have a financial interest related to this research? Indicate if the Principal Investigator has a financial interest. For more information regarding what constitutes a financial interest, please visit your IRB website. If an individual has a financial interest, complete and attach “FORM: Financial Interest Disclosure Form” to the Supporting Documents page of the SmartForm.
10. Study's department. Indicate the name of the Principal Investigator or Faculty Sponsor’s department for this study. Once chosen, saved and submitted, the department cannot be changed.
Type the department name in the space provided (a list will appear with options from which to select), or you can click the select option to search the list of available departments and schools. Use the “%” symbol to replace portions of a word of which may be unclear or possibly not spelled correctly. This is called a “wildcard” character.
If the department name does not appear on the list provided, contact estrhelp@harvard.edu for guidance on which department.
Once chosen, saved and submitted, the department cannot be changed during review. To change the department after a study is submitted (but before the first determination by the IRB):
1. Complete the Withdraw activity on the study in review
2. Click “Edit Study” to change the name of the department on the SmartForm
3. Click “Save” then “Exit” on the SmartForm menu
4. The PI must complete the Submit activity on the study to send it back to the IRB
11. Attach the Research Protocol or Relevant Request Form. Select and use the template provided on the SmartForm (or on your IRB website) that is appropriate for your project and the IRB to which you are submitting. Attach a copy of the completed protocol template, or if this application represents a request for a “Not Human Subjects Research determination”, attach “FORM: Non-Human Subjects Research Request Determination Form.” Of note, a research protocol is required for Exemption Requests. Be sure to name the file in a manner that identifies it as your protocol.
SmartForm Section: Funding Sources
On this page include any pending/awarded funding sources or financial support for this study. Leave questions 1 and 2 blank to indicate that there is no funding for this study. Reminder: If the funding status changes following IRB determination, submit a modification to this study.
1. List any grant proposal that has been submitted to the sponsored programs office (SPA or OSP), any proposal that was created in GMAS, or any federal or other sponsored funding for the study. Find and add all funding sources listed in the Harvard Grants Management Application Suite (GMAS) that are associated with this study.
To search for funding in the space provided, begin typing the grant project ID, the grant PI full name (first and last name), or the GMAS fund number and a list will appear with options from which to select.
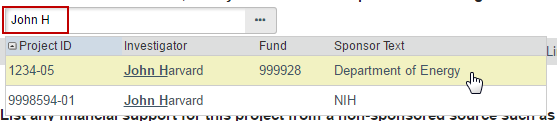
Begin If additional search options are needed, can click the ellipses […] button option to search the list of available funding sources. Use the “%” symbol to replace portions of a word of which may be unclear or possibly not spelled correctly. Click “Go” and select the correct funding source. Click “OK” on the pop up.
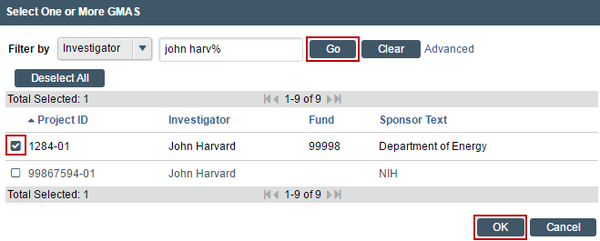
2. List any financial support for this project from a non-sponsored source such as a department, gift or Harvard program. List all other funding sources. Attach a complete copy of the funding application or agreement for these listed sources to the Supporting Documents page of the SmartForm, when applicable, and remove or black-out any salary information.
SmartForm Section: (Local) Study Team Members
The Study Team Members page of the SmartForm should include the name of individuals that a) have contact with human subjects, b) have access to data that is identifiable; or c) are responsible for the design, conduct, or reporting of the research. Take care to follow the instructions that display on the SmartForm page.
1. List study team members with an HUID. Click “Add” to include all team members who have an HUID.
Individuals can be selected from a list of names. Begin typing a piece of the person’s name in the space provided on the pop up, or you can click the “add” option to search the list of available names. Use the “%” symbol to replace portions of a name of which may be unclear or possibly not spelled correctly. This is called a “wildcard” character. Use the email address column for search or as a guide to selecting the correct person. If the correct person cannot be found via name or email, click the ellipses […]. Choose Filter by: UserID to search by HUID
If you would like a person on the study team to access ESTR, and his/her name does not appear in the search results; this individual must obtain an HUID. Please visit the ESTR support site Job Aids page for detailed instructions on how to obtain an HUID for use with ESTR.
Each of the individuals listed must complete human research training unless you clearly indicate that the individual is listed simply for access to this study record (in the Study Team Member details and via “Add comment” on the submission workspace).
Indicate whether each listed individual has a financial conflict of interest relating to the Human Research. For more information about what constitutes a conflict, please visit your IRB website. If an individual has a financial interest, complete “FORM: Financial Interest Disclosure Form” and attach it to item 2 on this page of the SmartForm.
2. Attach relevant study team documents. If an individual’s name does not appear in the search results available in question 1, attach a completed “FORM: Non-Harvard Study Personnel Form” This form should only list individuals, who were not able to be listed under item 1 in the Study Team Members section of the SmartForm. Each of the individuals listed must complete human research training. Individuals only listed on this form will not have access to ESTR.
Indicate whether each listed individual has a financial conflict of interest relating to the Human Research. For more information about what constitutes a conflict, please visit your IRB website. If an individual has a financial interest, complete “FORM: Financial Interest Disclosure Form” and attach it here.
| TIPS |
Study Team Members Page:
. |
SmartForm Section: Study Scope
1. Does the study involve the use of a drug in one or more persons other than use of an approved drug in the course of medical practice? Indicate if drugs, biologics, foods or dietary supplements are used in the Human Research.
SmartForm Sub-Section: Drugs
Identify all drugs, biologics, foods and dietary supplements (approved and unapproved) being used in the Human Research. For each, indicate whether it has an IND number and for those that do, ensure that the application includes one of the following: (a) sponsor protocol with the IND number; (b) communication from the sponsor with the IND number; or (c) communication from the FDA with the IND number. Additionally attach the following items:
> Investigator Brochure for each investigational drug involved in the Human Research.
> Current Package Insert. Submit for each marketed drug involved in the Human Research.
> Validation of IND#, e.g. FDA Approval letter or Sponsor Protocol
2. Does the study involve: (1) The use of a device in one or more persons that evaluates the safety or effectiveness of that device, or (2) Data regarding the use of a device on human specimens? Indicate if a device is used in the Human Research.
SmartForm Sub-Section: Devices
Identify all devices being evaluated for safety or effectiveness or as a comparator (approved and unapproved). For each, indicate whether it has an IDE number and for those that do, ensure that the application includes one of the following: (a) sponsor protocol with the IDE number; (b) communication from the sponsor with the IDE number; or (c) communication from the FDA with the IDE number. Indicate whether the device is being submitted under the “Abbreviated IDE requirements” in 21 CFR 812.2(b). Additionally attach the following items:
> Current Product Information, e.g., Device Manual. Submit for each investigational device involved in the Human Research.
> Validation of IND# or IDE#, e.g. FDA Approval letter or Sponsor Protocol
SmartForm Section: Local Research Location
Identify other research locations where the investigator will conduct or oversee the research. Indicate details as prompted on the research location details. For each location, the country is a required selection. If the location country is not available select the option “[country not listed]”.
Upload information for each location to the Local Site Documents page of the SmartForm, via the last document attachment prompt, when applicable.
SmartForm Sub-Section: Study Related Documents
Note: This page only displays if SmartForm Section: Basic Information question 4 is marked “Multi-Site/Collaborative” and SmartForm Section: Basic Information question 5 is marked “yes”; meaning that this is a study with sites, where at least one site will rely on Harvard’s review.
Important! This page of the SmartForm is intended to capture only documents that (1) have not been included in other sections of the SmartForm AND will be used at this site only.
1. Consent, Assent, Permission, and HIPAA Authorization Forms (English versions): Use the templates appropriate to your IRB to create and attach the following items. Visit your IRB website for additional instructions regarding how to create consent materials:
- Consent, Assent Forms, or Scripts including HIPAA Authorization Forms (as applicable). Consent/assent documents must include version date and/or version number. If any consent materials will be translated, attach the foreign language versions of materials to the last section of this page of the SmartForm.
- DHHS-approved sample consent document. Consent documents must include version date and/or version number.
If any of materials will be translated, attach the foreign language versions of materials to the last section on this page of the SmartForm.
2. Recruitment Materials (English versions): Each recruitment document, script, flyer, advertisement, etc. must include version date and/or version number. Guidance on what is appropriate to include and exclude within an advertisement can be found in “WORKSHEET: Advertisements” and/or “WORKSHEET: Payments.” Worksheets are available for reference on your IRB website or in the Library section of ESTR (linked at the top of the screen under the “IRB” heading). Advertising material must include version date and/or version number.
If any of materials will be translated, attach the foreign language versions of materials to the next section on this page of the SmartForm.
Attach other supporting files, naming each file as it should appear in the approval letter: Also, be sure to name the file itself in a manner that identifies it as associated with your study. The suggested best practice for file names: StudyNumber_DocumentName_VersionNumber
When uploading documents to this page, you are prompted to select the appropriate attachment category. Select the correct category when uploading the first version. Once chosen and saved, the attachment category cannot be changed. To change a category, a document must be removed and then re-uploaded with the new category.
Use the “other” category to attach non-document type attachments which must be part of the review (such as compressed/zip, video, audio, or htm file formats).
Items to attach to this section:
- Ancillary Approvals/Permissions. Submit approval letters or permissions from any additional office or organization reviewing this project (for example, a letter of support). If unavailable at the time of submission, plan to submit a copy to the IRB prior to implementing any study procedures.
- Debriefing Materials. If any of these materials will be translated, also submit a Translation Attestation Form.
- Research Location Information. If question 1 in the SmartForm Section: Study Scope indicated that there are research locations where the research will be conducted submit relevant approvals (such as a Community Advisory Board approval) or other information from each research location identified in the SmartForm. If unavailable at the time of submission, plan to submit a copy to the IRB prior to beginning any human subjects research at the location(s).
- Study Instruments/Tools. Including all data collection instruments, questionnaires, surveys, focus group discussion guides, or interview guides. Do not include Case Report Forms. Study documents must include version date and/or version number.
SmartForm Section: Local Site Documents
This page of the SmartForm is intended to capture documents that will be used by Harvard researchers. Ensure that the document versions attached here are relevant to for the specific site under review. Use the main study submission for Harvard documents and use the Site SmartForm, to attach site specific versions, only when appropriate.
1. Consent, Assent, Permission, and HIPAA Authorization Forms (English versions): Use the templates appropriate to your IRB to create and attach the following items. Visit your IRB website for additional instructions regarding how to create consent materials:
- Consent, Assent Forms, or Scripts including HIPAA Authorization Forms (as applicable). Consent/assent documents must include version date and/or version number. If any consent materials will be translated, attach the foreign language versions of materials to the last section of this page of the SmartForm.
- DHHS-approved sample consent document. Consent documents must include version date and/or version number.
If any of materials will be translated, attach the foreign language versions of materials to the last section on this page of the SmartForm.
2. Recruitment Materials (English versions): Each recruitment document, script, flyer, advertisement, etc. must include version date and/or version number. Guidance on what is appropriate to include and exclude within an advertisement can be found in “WORKSHEET: Advertisements” and/or “WORKSHEET: Payments.” Worksheets are available for reference on your IRB website or in the Library section of ESTR (linked at the top of the screen under the “IRB” heading). Advertising material must include version date and/or version number.
If any of materials will be translated, attach the foreign language versions of materials to the next section on this page of the SmartForm.
Attach other supporting files, naming each file as it should appear in the approval letter: Also, be sure to name the file itself in a manner that identifies it as associated with your study. The suggested best practice for file names: StudyNumber_DocumentName_VersionNumber
When uploading documents to this page, you are prompted to select the appropriate attachment category. Select the correct category when uploading the first version. Once chosen and saved, the attachment category cannot be changed. To change a category, a document must be removed and then re-uploaded with the new category.
Use the “other” category to attach non-document type attachments which must be part of the review (such as compressed/zip, video, audio, or htm file formats).
Items to attach to this section:
- Ancillary Approvals/Permissions. Submit approval letters or permissions from any additional office or organization reviewing this project (for example, a letter of support). If unavailable at the time of submission, plan to submit a copy to the IRB prior to implementing any study procedures.
- Data use agreements or other Agreements. Submit copies of any documents authorizing the use of data, or other contracts or agreements associated with the study.
- Debriefing Materials. If any of these materials will be translated, also submit a Translation Attestation Form.
- Research Location Information. If question 1 in the SmartForm Section: Study Scope indicated that there are research locations where the research will be conducted submit relevant approvals (such as a Community Advisory Board approval) or other information from each research location identified in the SmartForm. If unavailable at the time of submission, plan to submit a copy to the IRB prior to beginning any human subjects research at the location(s).
- Federal Department Requirements Checklists. Attach any required checklist associated with certain kinds of Federal funding (such as DOD, DOJ or EPA).
- Financial Interest Disclosure Form. Submit this form if the Principal Investigator self-identifies a financial conflict of interest related to the research on the SmartForm: Basic Information page. Forms for any other team members which self-identify a financial conflict of interest should be attached to the SmartForm: Study Team Members page.
- Foreign Language Documents. Submit all translated study materials.
- Funding Source Attachments. If not associated with GMAS and as applicable, submit a complete copy of the grant applications, subcontract, and/or any funding agreements regardless of funding source.
- Individual Investigator Agreement (IIA). When a non-Harvard collaborator will be covered under the Harvard IRB review and this person is not affiliated with another institution, submit this form here or attached a completed copy to the SmartForm: Study Team Members page.
- PI’s Current CV (ICH-GCP E6 Only). Submit a copy of the Principal Investigator’s current (signed/dated) CV only when required by sponsor to follow the “International Council on Harmonisation – Good Clinical Practice E6.”
- Radiation Safety Form. If the Human Research involves the use of approved or unapproved diagnostic or therapeutic radiation outside routine clinical practice, complete and submit “FORM: Radiation Safety.”
- Sponsor Protocol including DHHS-approved protocol. Submit the sponsor protocol, if applicable.
- Study Instruments/Tools. Including all data collection instruments, questionnaires, surveys, focus group discussion guides, or interview guides. Do not include Case Report Forms. Study documents must include version date and/or version number.
Upon selecting continue from this page, the browser will direct to the last page of the SmartForm where you will have the opportunity to select “Save & Exit” to return to the submission workspace.
