At any stage during the review process, the IRB may request clarifications to the study. Similarly, the official IRB determination may be that the study requires changes (or additional modifications) before research can begin. Both situations require the study staff to take similar actions. In either case, the PI, any PI proxy, and the study's primary contact will receive a system notification requesting either clarifications or modifications. The study also appears in My Inbox for each member of the study team.
Important! Any study team member can update the study and submit a response. Failure to respond promptly slows the review and approval process for your submission. In some cases, your submission may be rescheduled for review at a later IRB meeting because the committee requires your response before making a decision.
To view the details of the request and respond with the changes:
1. From My Inbox, click the name of the study to open it.
2. Locate the details of the request, as described here:
For Clarification Requested: In the Activity column under Clarification Requested, read the request details. If applicable, click the read more link to display the remaining text.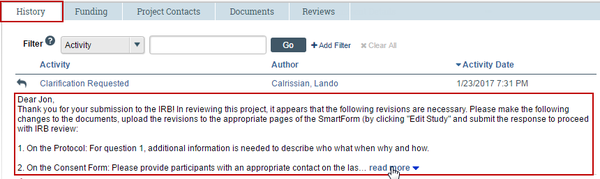
For Modifications Required: Click the letter link near the top of the page on the right side. The letter contains the modification requirement details.
3. Edit the study to incorporate changes as needed. For instructions, see Editing a Study.
Notes:
> In most cases, you can update all aspects of the study, including adding, updating, or removing attached documents.
> When updating a study document previously submitted to the IRB, revise it in tracked-changes format and replace the original document with the tracked-changes version. When the IRB approves the document, all tracked changes will be accepted and comments removed in the final version.
> If clarifications were requested during Committee Review, you cannot edit the study, and you see the View Study button instead. In that case, respond to the reviewer by commenting in the Submit Response form, as described in the next step.
4. Click Submit Response to return the study to the reviewers. 
Notes:
> The Submit Response form gives you space to type a point-by-point response to the requests and to attach a file. However, any permanent study information should be incorporated into the SmartForm itself.
> If clarifications were requested during committee review, you may be asked to make changes to the study after the review is complete.
> For an RNI submission, click Submit RNI Response instead.
5. Click OK. The study returns to the review process.
For information about completing an action plan for an RNI submission, see What to Include in a Reportable New Information SmartForm.