The Create Printable Attachment activity (available on all workspaces except Site or Site Modification) assists IRB staff with organizing materials for review. Completing this activity will generate one file pdf file of all submission attachments, for further formatting (if needed). This activity can only be conducted by IRB staff and committee Members.
To create a printable attachments:
1. Click Create Printable Attachments on the left![]()
2. A pop up window will appear during processing and will close automatically
3. View the “History” tab on the workspace to find the created files. Note that Projects which include IND/IDE attachments may not be able to generate a printable attachment file. 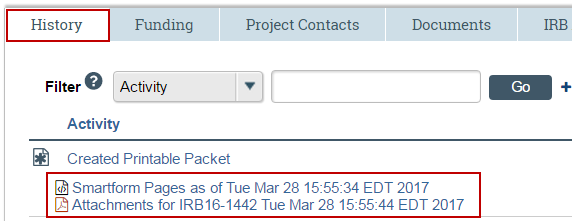
4. Click on the file to view and/or save to a separate space for further formatting. Note: The file available on the history represents the submission at a single point in time and are not updated when the SmartForm is updated.
5. To View or print the submission SmartForm, click the printer Version or View options on the left side of the wrokspace, as available.
Attachments File Contents
When clicking on the file entitled “Attachments for…” attachments appear in the following order (as applicable):
- Reports of New Information attachments: Only RNI attachments in the order they are attached
-
Initial Submission, Continuing Review, and Modification/Update attachments:
1. Protocol Attachments
2. External Site Attachments
3. Drug Attachments
3.1. Attachments for each Drug
3.2. IND Attachments
3.3. Other Drug Attachments
4. Device Attachments
4.1. Attachments for each Device
4.2. Other Device Attachments
5. Consent Forms, Assent Forms, HIPAA Authorization Materials
6. Supporting Documents, by category:
6.1. Ancillary Approvals/Permissions
6.2. Data use agreements or other Agreements
6.3. Debriefing Materials
6.4. External Site Information
6.5. Federal Department Requirements Checklists
6.6. Financial Interest Disclosure Form
6.7. Foreign Language Documents
6.8. Funding Source Attachments
6.9. Individual Investigator Agreement (IIA)
6.10. IRB Authorization Agreement (IAA) Request
6.11. Other
6.12. PI's Current CV (ICH-GCP E6 Only)
6.13. Protocol Attachment (if attached to the Supporting Documents page of the SmartForm)
6.14. Radiation Safety Form
6.15. Recruitment Materials/Advertisements
6.16. Sponsor Protocol including DHHS-approved protocol
6.17. Study Instruments/Tools
7. Study Personnel Forms (including any attached CITI documents)