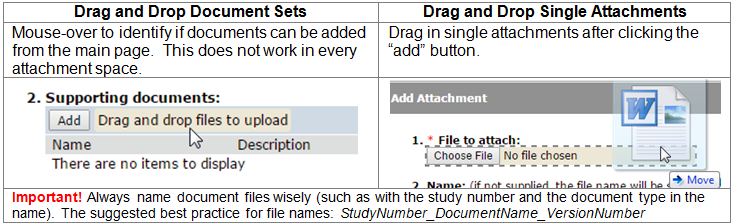
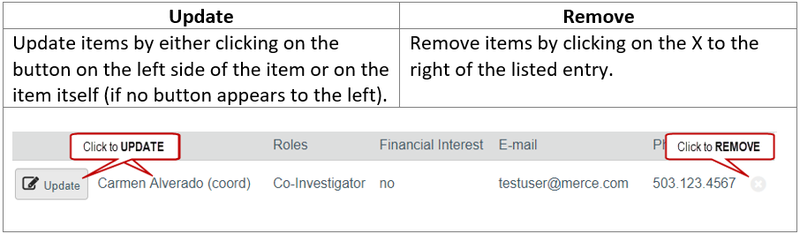
You can create a new study for IRB review by entering information into a series of online forms. The number of forms included may change based on the answers you provide. The forms tell you where to attach files to provide supporting information. The forms may contain required information identified by a red asterisk (*), you cannot proceed without providing that information. A required attachment to the Basic Information form is a Word document that describes the research: either the Protocol Template or the Not Human Subjects Research Determination Request. Additional documents should be attached to the forms where appropriate, such as recruiting, consenting and debriefing subject material. It is recommended that these documents be prepared prior to creating the new study; templates and guidance are available on your IRB website.
The simplest approach is to follow the forms in order, answering the questions and clicking Continue to save your information and move to the next form. When you reach the end of the series of forms, click the Save & Exit button.
To create a new study for review:
-
From My Inbox or IRB pages (linked in the top menu), click Create New Study.
-
Fill in the applicable boxes, answer the questions, and attach appropriate documents.
-
Click Continue to move to the next form.

-
Click the ‘jump to’ menu to go to a specific section.
-
To save or exit the SmartForm at any time, click on “Save” and “Exit” button in the top toolbar. Exit will take you to that submission’s workspace.

-
When you reach the final page, click Save & Exit to exit the study.
You can continue to edit the study until you submit it for review. See Editing a Submission.
Important! The study has not been submitted for review yet. For instructions, see Submitting the Study for Review.
| TIPS |
|