You may need to modify a study's documents when:
> The IRB requires changes prior to approval.
> Submitting a modification to an approved study.
To change documents prior to study approval:
Note: These steps also apply if the IRB decision was modifications required, disapproved, or deferred.
1. From My Inbox, click the name of the submission to open the workspace
2. Click the Documents tab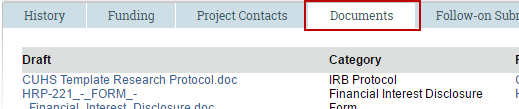
3. Click the document in the Draft column and save it to your computer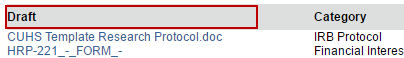
4. Open the saved document and make edits (Enable the Tracked Changes feature as needed. When the IRB approves the document, all tracked changes will be accepted and comments removed in the final version.)
5. When finished with the changes, save the revised document
6. Go to the submission workspace and click Edit Study on the left. For Modifications or Continuing Reviews, click Edit Submission.
7. Navigate to the location of the current document within the SmartForm by clicking Continue on the right
8. Replace the original study document with the revised version by clicking “Update” beside the document on the SmartForm, or use other controls to make document changes.
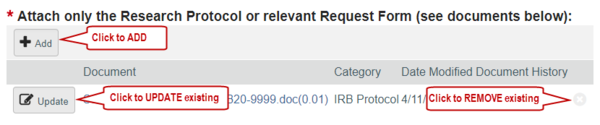
9. Complete any further updates and submit the changes for further review. See the section Editing a Submission for further details.
To change documents on an approved study:
1. Click IRB in the top navigation area and select the Active tab.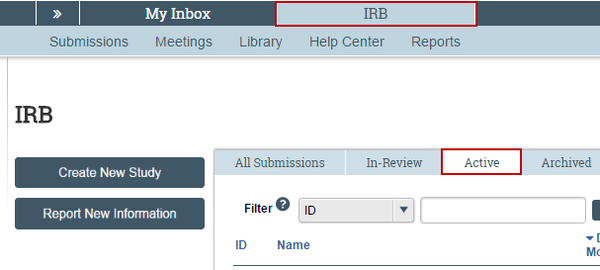
2. Click the name of the approved study.
3. Click the Documents tab.
4. Click the document in the Draft column and save it to your computer. In some cases, this document may contain previously approved tracked changes and comments. To update contents content match the current final-approved version, use the review features in Word to accept all the changes and remove any comments. Use this clean document as a starting point for your revisions.
5. Open the saved document and make edits (Enable the Tracked Changes feature as needed. When the IRB approves the document, all tracked changes will be accepted and comments removed in the final version.)
6. When finished with the changes, save the revised document
7. Go to the approved study workspace and create a modification to update the SmartForm and submit the proposed changes for IRB review. See the section Creating a Follow-On Submission after Study Approval for further details.